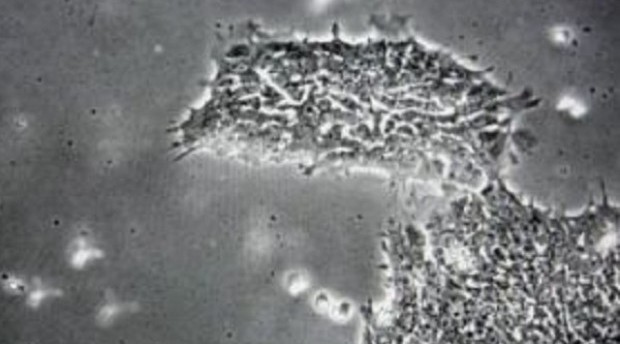
MANILA, Philippines – Health authorities warned the public anew on Saturday about health facilities and medical practitioners offering unauthorized stem cell therapy and products.
In an advisory, the Food and Drug Administration said that to date, “not one stem cell or human cells, tissues, and cellular and tissue-based products (HCT/Ps) that applied for registration has been registered by the FDA for compassionate or clinical trial use, or general use.”
“The use of HCT/Ps without the authorization or permission by the FDA is considered illegal,” it said, reminding hospitals and health facilities of the provisions of the FDA Act of 2009, which prohibits the manufacture, use, advertisement or sponsorship of unregistered health products.
“This warning extends to all unlicensed practitioners from other countries and to tourists who visit the Philippines for leisure and medical needs,” the advisory added.
According to FDA acting director general Kenneth Hartigan Go, the FDA recognizes only hematopoietic (pertaining to the formation and development of blood cells) stem cell transplantation, corneal resurfacing with limbal stem cells and skin regeneration with epidermal stem cells “as generally accepted standards of health care procedures.”
“If health institutions are doing these three procedures, they can continue doing them because those are allowed.” Go said, adding that the efficacy of the use of stem cells for the treatment of other diseases, such as diabetes, cancer and autism, among others, has yet to be proven.
Go noted that while many spa centers and salons are advertising stem cell therapy and products, none of them has the approval of the health agency.
“As of now we have not accredited any health facility offering stem cell therapy yet,” Go said.
Several facilities had applied for accreditation but Go said many of these were asked to correct their “deficiencies.”
Submission of an application, he stressed, did not necessarily result in automatic approval.
The FDA guidelines require that all stem cell and cellular-based treatments offered in the country should first pass the agency’s standards for safety, efficacy and quality.
“All practitioners that offer stem cell therapy to their patients as clinical trial or for compassionate use are required to seek FDA approval or permission to ensure compliance to good clinical practice, for the protection of the health and welfare of the patients,” Go said.
He also urged the public to report to the authorities those who continue to undertake stem cell therapy procedures, or who mislead their patients on the use of HCT/Ps as standard care for a specific condition, as well as any adverse events following the use of HCT/Ps.
The FDA said it had been continuously collaborating with the Department of Health’s Bureau of Health Facilities and Services in accrediting facilities that deal with human cells, tissues, and cellular and tissue-based products (HCT/Ps), laboratory and therapeutic activities or services.
RELATED STORIES
The furor over fresh-cell therapy (which is NOT stem cell therapy)
DDB chief sues doctors for fake stem cell treatment
Labor chief airs warning vs stem cell therapy